Management
On this page:
- Introduction
- Role of Weeds in the Management of TSW
- Resistant Tomato Cultivars for Managing TSW
- Resistant Pepper Cultivars for Managing TSW
- Use of Reflective Plastic Mulch for Managing TSW
- Use of Plant Activators for Managing TSW
- Control of Thrips Vectors with Insecticide
- Forecasting Thrips and TSW
- References
Introduction
 Management of tomato spotted wilt is best approached though the use of multiple tactics. This is because the use of a single tactic, even a very effective tactic like host plant resistance to the virus, runs the risk of that tactic failing over time. The most notable example of this has been the over reliance on pesticides which quickly leads to pesticide resistance in the pest organism. In the following section we highlight the management topics and specific management tactics that are critical for understanding and managing thrips and the Tomato spotted wilt virus (TSWV) that it transmits. These include the role of weeds in the spread of TSWV, TSWV resistance in tomato and pepper cultivars, the use of reflective, metallic coated plastic mulch in reducing the incidence of this disease, the use of plant activators for reducing disease symptoms, control of thrips vectors with insecticide and forecasting thrips and TSWV disease incidence as a means of establishing your risk. Tomato spotted wilt risk calculations for pepper and tomato are provided at the end to make a quick assessment of your best approach to managing this disease problem. All of the recommendations listed here have been field tested and viewed at field days, like the one pictured above held at the UGA Tifton campus.
Management of tomato spotted wilt is best approached though the use of multiple tactics. This is because the use of a single tactic, even a very effective tactic like host plant resistance to the virus, runs the risk of that tactic failing over time. The most notable example of this has been the over reliance on pesticides which quickly leads to pesticide resistance in the pest organism. In the following section we highlight the management topics and specific management tactics that are critical for understanding and managing thrips and the Tomato spotted wilt virus (TSWV) that it transmits. These include the role of weeds in the spread of TSWV, TSWV resistance in tomato and pepper cultivars, the use of reflective, metallic coated plastic mulch in reducing the incidence of this disease, the use of plant activators for reducing disease symptoms, control of thrips vectors with insecticide and forecasting thrips and TSWV disease incidence as a means of establishing your risk. Tomato spotted wilt risk calculations for pepper and tomato are provided at the end to make a quick assessment of your best approach to managing this disease problem. All of the recommendations listed here have been field tested and viewed at field days, like the one pictured above held at the UGA Tifton campus.
Role of Weeds in the Management of TSW
 Weeds play a critical role in the epidemiology of TSWV by serving as reservoirs for the virus and as reproductive hosts for the thrips vectors. Without endemic infection within local weed populations, TSWV incidence in crop plants would remain low. Reducing virus incidence through weed host management is a compelling idea, but it is one that is more complex than it might at first seem. At any given location, virus spread depends on multiple environmental factors and their impacts on the biology of host plants and the thrips vectors. Winter annual weeds, such as cudweed pictured in Fig. 1, are often considered of primary importance in virus movement; nevertheless, perennial, biennial and summer annuals also contribute to TSWV spread. Because only immature thrips can acquire the virus, only plants that serve as both host to the virus and reproductive host to thrips are important in the spread of the pathogen. The spread of TSWV from weed hosts to crops is dependent on the abundance of infected weeds and their proximity to susceptible crop plants. Thrips abundance and dispersal are the other key components of virus spread, and they are highly influenced by environmental factors such as temperature, rainfall and host plant suitability.
Weeds play a critical role in the epidemiology of TSWV by serving as reservoirs for the virus and as reproductive hosts for the thrips vectors. Without endemic infection within local weed populations, TSWV incidence in crop plants would remain low. Reducing virus incidence through weed host management is a compelling idea, but it is one that is more complex than it might at first seem. At any given location, virus spread depends on multiple environmental factors and their impacts on the biology of host plants and the thrips vectors. Winter annual weeds, such as cudweed pictured in Fig. 1, are often considered of primary importance in virus movement; nevertheless, perennial, biennial and summer annuals also contribute to TSWV spread. Because only immature thrips can acquire the virus, only plants that serve as both host to the virus and reproductive host to thrips are important in the spread of the pathogen. The spread of TSWV from weed hosts to crops is dependent on the abundance of infected weeds and their proximity to susceptible crop plants. Thrips abundance and dispersal are the other key components of virus spread, and they are highly influenced by environmental factors such as temperature, rainfall and host plant suitability.
What determines the abundance of virus infected weeds?
TSWV has a host range of over 800 plant species, and the landscape of the southeastern US contains numerous competent weedy and cultivated hosts. The small field size and diverse landscape typical of southeastern agriculture ensures that most crop fields will be close to areas where infected weeds are plentiful, and many of these weeds also support reproducing populations of thrips that transmit the virus. Because of this, reducing weed density in field borders may not have a major impact on TSWV movement because thrips can migrate from nearby lands that are not directly adjacent to the field.
What determines thrips abundance and dispersal from weeds?
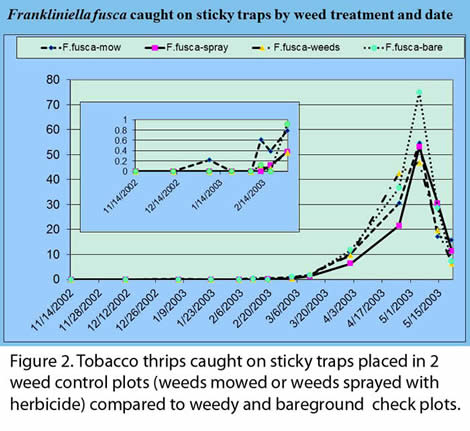 Large populations of virus infected thrips dispersing from overwintering sites during periods when susceptible crop plants are present can result in severe disease pressure and economic loss. Heavy rain and prolonged periods of drizzle in early spring have both been shown to kill thrips, reducing overall populations. On the other hand, warm spring temperatures provide favorable conditions for rapid development of thrips populations on winter annual weeds and result in increased thrips abundance. Large vector populations will not result in significant virus spread unless susceptible plants are present during dispersal. Growers cannot control rainfall or spring time temperatures, but they can avoid planting susceptible crops during periods of peak thrips dispersal. Thrips dispersal is triggered by declining host plant suitability. In the case of winter annuals, senescence occurs naturally with increasing spring temperatures or it can be brought about by agricultural practices such as herbicide application. In a 2003 study at Tifton (Fig. 2), the mowing of weeds in the spring around tomatoes prior to transplant caused increased numbers of dispersing thips on traps (small inset on Fig. 2) and increased incidence of Tomato spotted wilt in the adjacent tomato crop. Spraying herbicides tended to decrease thrips numbers on traps compared to bare ground and weedy check plots. Thus, the choice and timing of weed control can decrease or increase the tomato spotted wilt incidence in the crop, so care should be taken. For example, application of glyphosate (RoundUp©), a slow-acting herbicide, to winter weeds can increase thrips dispersal for three weeks post application, and application of paraquat (Grammoxone ©), a fast-acting herbicide, can increase thrips dispersal for at least one week. If herbicide must be applied to winter weeds near tomato spotted wilt susceptible crops, glyphosate should be applied four weeks prior to susceptible crop transplant and paraquat should be applied 2-3 weeks prior to transplant. Disking of weeds is not known to increase thrips dispersal and can be performed in areas adjacent to susceptible transplants. Although prevention of increased thrips dispersal by appropriate timing of herbicide application can minimize risk of TSWV spread, you need to be aware that the use of herbicide does not come close to eliminating TSWV infection risk because thrips can disperse long distances. In fact, the cost of weed control over and above what is done with normal, good vegetable production practices will likely not be cost effective. Therefore excessive wide-spread weed control is not recommended.
Large populations of virus infected thrips dispersing from overwintering sites during periods when susceptible crop plants are present can result in severe disease pressure and economic loss. Heavy rain and prolonged periods of drizzle in early spring have both been shown to kill thrips, reducing overall populations. On the other hand, warm spring temperatures provide favorable conditions for rapid development of thrips populations on winter annual weeds and result in increased thrips abundance. Large vector populations will not result in significant virus spread unless susceptible plants are present during dispersal. Growers cannot control rainfall or spring time temperatures, but they can avoid planting susceptible crops during periods of peak thrips dispersal. Thrips dispersal is triggered by declining host plant suitability. In the case of winter annuals, senescence occurs naturally with increasing spring temperatures or it can be brought about by agricultural practices such as herbicide application. In a 2003 study at Tifton (Fig. 2), the mowing of weeds in the spring around tomatoes prior to transplant caused increased numbers of dispersing thips on traps (small inset on Fig. 2) and increased incidence of Tomato spotted wilt in the adjacent tomato crop. Spraying herbicides tended to decrease thrips numbers on traps compared to bare ground and weedy check plots. Thus, the choice and timing of weed control can decrease or increase the tomato spotted wilt incidence in the crop, so care should be taken. For example, application of glyphosate (RoundUp©), a slow-acting herbicide, to winter weeds can increase thrips dispersal for three weeks post application, and application of paraquat (Grammoxone ©), a fast-acting herbicide, can increase thrips dispersal for at least one week. If herbicide must be applied to winter weeds near tomato spotted wilt susceptible crops, glyphosate should be applied four weeks prior to susceptible crop transplant and paraquat should be applied 2-3 weeks prior to transplant. Disking of weeds is not known to increase thrips dispersal and can be performed in areas adjacent to susceptible transplants. Although prevention of increased thrips dispersal by appropriate timing of herbicide application can minimize risk of TSWV spread, you need to be aware that the use of herbicide does not come close to eliminating TSWV infection risk because thrips can disperse long distances. In fact, the cost of weed control over and above what is done with normal, good vegetable production practices will likely not be cost effective. Therefore excessive wide-spread weed control is not recommended.
The role of perennials and summer annuals in TSWV spread
 Though winter annual weeds are a significant factor determining the extent of TSWV spread to crops, summer annual and perennial weeds can play an important role by serving as reservoirs for the virus when winter annual weeds and crop hosts are not present. Perennial plants can remain positive for TSWV for years after initial infection, and they can harbor large populations of reproducing thrips. In years when winter annual weeds senesce before susceptible crop plants are available, summer annual weeds can serve as a bridge to maintain virus moving out of winter hosts. In addition, abandoned vegetable fields (Fig. 3) can maintain both thrips and inoculum for subsequent planting of susceptible vegetable crops.
Though winter annual weeds are a significant factor determining the extent of TSWV spread to crops, summer annual and perennial weeds can play an important role by serving as reservoirs for the virus when winter annual weeds and crop hosts are not present. Perennial plants can remain positive for TSWV for years after initial infection, and they can harbor large populations of reproducing thrips. In years when winter annual weeds senesce before susceptible crop plants are available, summer annual weeds can serve as a bridge to maintain virus moving out of winter hosts. In addition, abandoned vegetable fields (Fig. 3) can maintain both thrips and inoculum for subsequent planting of susceptible vegetable crops.
Author: Mark Abney
Resistant Tomato Cultivars for Managing TSW
 When Tomato spotted wilt (TSW) disease epidemics broke out in the 1990’s in tomato growing areas in northern Florida, southern Georgia, Alabama, South Carolina and North Carolina, growers were immediately interested in acquiring tomato cultivars that were resistant because the disease was so severe (Fig. 1). Host plant resistance, where available, has traditionally given growers one of the most powerful and economical tools for managing viral diseases in vegetable crops. During the 1990’s, a TSW-resistant tomato ‘Stevens’, was found to be highly resistant to TSW in Florida and Georgia field tests, but it proved to be less productive (less weight of marketable fruit per plant) than the widely planted TSW-susceptible tomato hybrid ‘FL 47’ at that time. Even so, this was a start. By 1998 the tomato, BHN444, was released that had equally high levels of TSW resistance and produced much more marketable weight per plant. Soon afterwards, commercial TSW-resistant hybrids (Fig. 2) began appearing on the market and the development of the most effective management tool for TSWV was well underway. Since this is currently our main tactic for TSWV control in tomato, it is important to understand from where this resistance come, what other types of resistance occur, and how stable resistance can be over time.
When Tomato spotted wilt (TSW) disease epidemics broke out in the 1990’s in tomato growing areas in northern Florida, southern Georgia, Alabama, South Carolina and North Carolina, growers were immediately interested in acquiring tomato cultivars that were resistant because the disease was so severe (Fig. 1). Host plant resistance, where available, has traditionally given growers one of the most powerful and economical tools for managing viral diseases in vegetable crops. During the 1990’s, a TSW-resistant tomato ‘Stevens’, was found to be highly resistant to TSW in Florida and Georgia field tests, but it proved to be less productive (less weight of marketable fruit per plant) than the widely planted TSW-susceptible tomato hybrid ‘FL 47’ at that time. Even so, this was a start. By 1998 the tomato, BHN444, was released that had equally high levels of TSW resistance and produced much more marketable weight per plant. Soon afterwards, commercial TSW-resistant hybrids (Fig. 2) began appearing on the market and the development of the most effective management tool for TSWV was well underway. Since this is currently our main tactic for TSWV control in tomato, it is important to understand from where this resistance come, what other types of resistance occur, and how stable resistance can be over time.
From where does TSWV resistance come?
Some of the earliest resistance work was reviewed by Stevens et al. (1992) who reported that these genes were derived from S. pimpinellifolium. Finlay (1953) tested resistance sources against 10 strains in Australia and identified two resistance alleles at the Sw-1 locus (Sw-1a, Sw-1b ) and three recessive resistance genes; sw-2, sw-3, and sw-4. According to the gene list published in 1959 the cultivar ‘Pearl Harbor’ has Sw-1a and ‘Rey de los Tempranos’ has Sw-1b and the three recessive genes (Clayberg et al., 1959).  These genes were strain specific in their effects. Paterson et al. (1989) tested the resistant sources of Finlay and found them to be susceptible to isolates in Arkansas. In 1986 resistance was reported in the South African cultivar ‘Stevens’ with resistance being introgressed from L. peruvianum (van Zijl et al, 1986). The resistant accession used in ‘Stevens’ is not known for sure but it may be PI 128654 (see Stevens et al., 1992). Stevens et al. (1992) determined that the resistance from ‘Stevens’ was conferred by a single dominant gene, named it Sw-5, and found it not to be race specific. Boiteux and Giordano (1993) found Sw-5 also provided resistance to the Tospoviruses chlorotic spot (TCS) and Groundnut ring spot (GRS). The Sw-5 gene has been mapped to the long arm of chromosome 9 between CT71 and CT220 (Stevens et al., 1995; Brommonschenkel and Tanksley, 1997; Folkertsma, et al., 1999). A co-dominant SCAR marker linked tightly to the Sw-5 locus was developed from RAPD primer UBC#421 (Stevens et al., 1996) was widely used by tomato breeders around the world and Sw-5 has now been deployed in a number of cultivars.
These genes were strain specific in their effects. Paterson et al. (1989) tested the resistant sources of Finlay and found them to be susceptible to isolates in Arkansas. In 1986 resistance was reported in the South African cultivar ‘Stevens’ with resistance being introgressed from L. peruvianum (van Zijl et al, 1986). The resistant accession used in ‘Stevens’ is not known for sure but it may be PI 128654 (see Stevens et al., 1992). Stevens et al. (1992) determined that the resistance from ‘Stevens’ was conferred by a single dominant gene, named it Sw-5, and found it not to be race specific. Boiteux and Giordano (1993) found Sw-5 also provided resistance to the Tospoviruses chlorotic spot (TCS) and Groundnut ring spot (GRS). The Sw-5 gene has been mapped to the long arm of chromosome 9 between CT71 and CT220 (Stevens et al., 1995; Brommonschenkel and Tanksley, 1997; Folkertsma, et al., 1999). A co-dominant SCAR marker linked tightly to the Sw-5 locus was developed from RAPD primer UBC#421 (Stevens et al., 1996) was widely used by tomato breeders around the world and Sw-5 has now been deployed in a number of cultivars.
How stable is TSW resistance?
 Even the best sources of resistance can falter under high disease pressure, which is the case with Sw-5 (Rosello et al., 1997; 1998). Furthermore, strains virulent on Sw-5 cultivars have been reported in Hawaii (Cho et al., 1996), South Africa (Thompson and van Zijl, 1996), and Australia (Latham and Jones, 1998). If such strains appear in tomato production regions the resistance in cultivars based on Sw-5 could be rendered useless. Thus, it is important to have other resistance available. Rosello et al. (1999) used mechanical and thrips inoculation to screen L. peruvianum and L. chilense accessions and found useful resistance in L. peruvianum accessions PI 126935, PI 126944, CIAPAN 16, PE-18, and CIAPAN 17. Earlier, Rosello et al. (1998) reported resistance gene Sw-6 was introgressed from L. peruvianum accession PE-18. The level of resistance of Sw-6 was not as high as that provided by Sw-5. In later work, the Sw-6 gene present in line UPV 1 was determined to be allelic to Sw-5 but in heterozygous condition had a higher resistance level (Rosello et al., 2001). Other sources of resistance have been reported including a recovery type of resistance derived from L. chilense accession LA 1938 (Canady et al., 2001). Furthermore, this resistance has not been overcome by the Hawaiian strains that overcome Sw-5 (Stevens, personal communication). The inheritance of this resistance to strains that do and do not overcome Sw-5 needs to be elucidated and the development of molecular markers linked to the resistance gene(s) will greatly facilitate breeding progress with this material. A genetically engineered possibility for spotted wilt resistance was demonstrated in tomato and tobacco plants transformed with the nucleocapsid protein (N) gene from the virus is inserted in either sense or antisense orientations (Kim et al., 1994). Another approach to provide broad spectrum resistance was to combine transgenic resistance with Sw-5 resistance (Gubba et al., 2002). Fortunately over more than a decade, Sw-5resistance held up very well in the Southeastern USA during field testing from 2002 to 2012.
Even the best sources of resistance can falter under high disease pressure, which is the case with Sw-5 (Rosello et al., 1997; 1998). Furthermore, strains virulent on Sw-5 cultivars have been reported in Hawaii (Cho et al., 1996), South Africa (Thompson and van Zijl, 1996), and Australia (Latham and Jones, 1998). If such strains appear in tomato production regions the resistance in cultivars based on Sw-5 could be rendered useless. Thus, it is important to have other resistance available. Rosello et al. (1999) used mechanical and thrips inoculation to screen L. peruvianum and L. chilense accessions and found useful resistance in L. peruvianum accessions PI 126935, PI 126944, CIAPAN 16, PE-18, and CIAPAN 17. Earlier, Rosello et al. (1998) reported resistance gene Sw-6 was introgressed from L. peruvianum accession PE-18. The level of resistance of Sw-6 was not as high as that provided by Sw-5. In later work, the Sw-6 gene present in line UPV 1 was determined to be allelic to Sw-5 but in heterozygous condition had a higher resistance level (Rosello et al., 2001). Other sources of resistance have been reported including a recovery type of resistance derived from L. chilense accession LA 1938 (Canady et al., 2001). Furthermore, this resistance has not been overcome by the Hawaiian strains that overcome Sw-5 (Stevens, personal communication). The inheritance of this resistance to strains that do and do not overcome Sw-5 needs to be elucidated and the development of molecular markers linked to the resistance gene(s) will greatly facilitate breeding progress with this material. A genetically engineered possibility for spotted wilt resistance was demonstrated in tomato and tobacco plants transformed with the nucleocapsid protein (N) gene from the virus is inserted in either sense or antisense orientations (Kim et al., 1994). Another approach to provide broad spectrum resistance was to combine transgenic resistance with Sw-5 resistance (Gubba et al., 2002). Fortunately over more than a decade, Sw-5resistance held up very well in the Southeastern USA during field testing from 2002 to 2012.
Commercially available TSW-resistant tomato.
 The currently available TSW-resistant tomato cultivars do an excellent job of preventing the stunting plants caused by early infection of TSW, drastically increasing yields in a TSW epidemic year, and reducing the irregular ripened fruit commonly seen in susceptible tomato hybrids such as FL 47 (Fig. 3). Over the last decade of field testing at the University of Florida and the University of Georgia, a number of commercial tomato cultivars have been demonstrated to be highly resistant to the strain of TSW that we typically have in the Southeast and good quality crops. The breading program in Florida continues to produce new lines of TSW-resistant tomatoes with new genes for resistance. A list of round type tomato cultivars that have been confirmed resistant to TSW in replicated field trials in the Southeast are listed in the list below.
The currently available TSW-resistant tomato cultivars do an excellent job of preventing the stunting plants caused by early infection of TSW, drastically increasing yields in a TSW epidemic year, and reducing the irregular ripened fruit commonly seen in susceptible tomato hybrids such as FL 47 (Fig. 3). Over the last decade of field testing at the University of Florida and the University of Georgia, a number of commercial tomato cultivars have been demonstrated to be highly resistant to the strain of TSW that we typically have in the Southeast and good quality crops. The breading program in Florida continues to produce new lines of TSW-resistant tomatoes with new genes for resistance. A list of round type tomato cultivars that have been confirmed resistant to TSW in replicated field trials in the Southeast are listed in the list below.
| Cultivar | Plant Source |
| Quincy | Seminis |
| BHN 640 | BHN/Siegers |
| Crista | Harris Moran/Clifton |
| Bella Rosa | Sakata/Siegers |
| Amelia | Harris Moran/Clifton |
| Talladega | Syngenta |
| Red Defender | Harris Moran |
| BHN 444 | BHN/Siegers |
| Redline | Syngenta |
| BHN 602 | BHN/Siegers |
| Top Gun | Twilley |
| Inbar | Hazera |
| Tycoon | Hazera |
| Carson | Hazera |
| Tribeca | Vilmorin |
| Mountain Glory | Siegers |
| Fletcher | Reimer Seeds |
| Finishline | Syngenta |
| Tous 91 | Hazera |
| Nico | Harris Moran |
| Tribute | Sakata |
There are also Roma or plum-type tomato lines with resistance to TSW including: Picus (Seminis), Rubia (Sakata), Muriel, BHN 685 (Siegers), Hedvig, Galilea, and Shanty (Hazera). All of these are excellent producers and provide a high quality tomato under conditions in the southeastern USA.
Authors: J.W. Scott, S. Olson, D. Riley
Resistant Pepper Cultivars for Managing TSW
 Tomato spotted wilt (TSW) disease epidemics have been economically important in peppers since the 1990’s in the Southeast, as has been the case in tomato (Gitaitis et al. 1998), but it is interesting that the intensity of the epidemics in pepper seem to vary across the region and time. For example, bare ground plantings of pepper in the pepper growing areas of northern Florida and southern Georgia experienced severe disease pressure (example in Fig. 1). However, by the late 1990’s much of the acreage was planted on black plastic mulch and treated pre-transplant with imidacloprid and tended to have lower levels of the disease. As disease pressure continued to grow in plantings of susceptible tomato cultivars, the disease pressure in pepper grown on plastic mulch in this region consistently remained at a level lower than that seen in susceptible tomato on black plastic mulch. In North Carolina, no such difference was evident over time and TSW-susceptible pepper continues to experience significant disease pressure. Host plant resistance to TSW in pepper gives growers an economical tool for greatly reducing the risk of TSW disease without incurring much more production costs. One of the earliest commercial pepper cultivars available to growers in the Southeast was ‘Stilleto’ from Rogers Seed Company. This pepper was found to be highly resistant to TSW in Florida and Georgia field tests, but it proved to be less productive (less weight of marketable fruit per plant) than other widely planted TSW susceptible peppers at that time. By 2002 the pepper ‘Heritage’, which has equally high levels of TSW resistance and produces much more marketable weight per plant, was released by Harris Moran Seed Company. Soon afterwards, commercial TSW-resistant hybrids were being field-tested (Fig. 2) and highly productive cultivars became widely available. TSW-resistant pepper is now likely to provide the most stable solution to this problem over the long term (Genda et al., 2008). It is important to understand where this resistance comes from and how stable resistance can be over time.
Tomato spotted wilt (TSW) disease epidemics have been economically important in peppers since the 1990’s in the Southeast, as has been the case in tomato (Gitaitis et al. 1998), but it is interesting that the intensity of the epidemics in pepper seem to vary across the region and time. For example, bare ground plantings of pepper in the pepper growing areas of northern Florida and southern Georgia experienced severe disease pressure (example in Fig. 1). However, by the late 1990’s much of the acreage was planted on black plastic mulch and treated pre-transplant with imidacloprid and tended to have lower levels of the disease. As disease pressure continued to grow in plantings of susceptible tomato cultivars, the disease pressure in pepper grown on plastic mulch in this region consistently remained at a level lower than that seen in susceptible tomato on black plastic mulch. In North Carolina, no such difference was evident over time and TSW-susceptible pepper continues to experience significant disease pressure. Host plant resistance to TSW in pepper gives growers an economical tool for greatly reducing the risk of TSW disease without incurring much more production costs. One of the earliest commercial pepper cultivars available to growers in the Southeast was ‘Stilleto’ from Rogers Seed Company. This pepper was found to be highly resistant to TSW in Florida and Georgia field tests, but it proved to be less productive (less weight of marketable fruit per plant) than other widely planted TSW susceptible peppers at that time. By 2002 the pepper ‘Heritage’, which has equally high levels of TSW resistance and produces much more marketable weight per plant, was released by Harris Moran Seed Company. Soon afterwards, commercial TSW-resistant hybrids were being field-tested (Fig. 2) and highly productive cultivars became widely available. TSW-resistant pepper is now likely to provide the most stable solution to this problem over the long term (Genda et al., 2008). It is important to understand where this resistance comes from and how stable resistance can be over time.
Where does TSW resistance in pepper come from?
 Black et al. (1991) identified TSW-resistance from various accessions of Capsicum species that functioned through a hypersensitive response. Subsequently, the responsible TSW-resistant gene was characterized and named Tsw(Boiteux and de Avila, 1994; Boiteux et al., 1993; Boiteux 1995; Costa et al., 1995; Moury et al., 1997). This gene has since been introgressed into commercial lines to create TSW-resistant pepper cultivars (Black et al., 1996; Roggero et al., 2002; Persley et al., 2006). There have been several reports of resistance breaking TSW virus isolates world-wide (Boiteux and Nagata, 1992; Roggero et al., 2002; Margaria et al., 2004; Sharman and Persley, 2006) including in Louisiana (U.S.) (Hobbs et al., 1994). Most resistant cultivars of Capsicum chinensisJacq that are available for commercial pepper production have the Tsw gene (Roggero et al., 2002; Persley et al., 2006). Variation in the TSW virus isolate and plant phenotypes may affect performance of resistant pepper cultivars with the Tsw gene (Sharman and Persley, 2006). Pepper plants can become more resistant to TSW as they age (Beaudoin et al. 2009). In addition, temperature and gene dosage may influence the hypersensitive response of Tsw gene (Roggero et al., 1996; Moury et al., 1998; Soler et al., 1998). Recently, evidence for specific changes in the TSW virus has been reported that allow the pathogen to overcome resistance (Lopez et al. 2011).
Black et al. (1991) identified TSW-resistance from various accessions of Capsicum species that functioned through a hypersensitive response. Subsequently, the responsible TSW-resistant gene was characterized and named Tsw(Boiteux and de Avila, 1994; Boiteux et al., 1993; Boiteux 1995; Costa et al., 1995; Moury et al., 1997). This gene has since been introgressed into commercial lines to create TSW-resistant pepper cultivars (Black et al., 1996; Roggero et al., 2002; Persley et al., 2006). There have been several reports of resistance breaking TSW virus isolates world-wide (Boiteux and Nagata, 1992; Roggero et al., 2002; Margaria et al., 2004; Sharman and Persley, 2006) including in Louisiana (U.S.) (Hobbs et al., 1994). Most resistant cultivars of Capsicum chinensisJacq that are available for commercial pepper production have the Tsw gene (Roggero et al., 2002; Persley et al., 2006). Variation in the TSW virus isolate and plant phenotypes may affect performance of resistant pepper cultivars with the Tsw gene (Sharman and Persley, 2006). Pepper plants can become more resistant to TSW as they age (Beaudoin et al. 2009). In addition, temperature and gene dosage may influence the hypersensitive response of Tsw gene (Roggero et al., 1996; Moury et al., 1998; Soler et al., 1998). Recently, evidence for specific changes in the TSW virus has been reported that allow the pathogen to overcome resistance (Lopez et al. 2011).
Commercially available TSWV-resistant pepper.
Commercially available lines of pepper were field tested for resistance to Tomato spotted wilt (TSW) virus, a Tospovirus (Bunyaviridae), for 5 years (2006 to 2010) at the Coastal Plain Experiment Station at Tifton, GA USA and 2 years (2009 to 2010) at North Carolina. Selected cultivars were transplanted each year into four randomized complete block plots consisting of standard, black plastic mulch beds on drip irrigation. These tests were conducted in the spring of each year when the incidence of TSW tended to be highest. Also, presence of thrips vectors was monitored using beat cup sampling of foliage and flower samples. Yield was estimated according to USDA pepper grades and the percent TSW symptomatic fruit was assessed. Pepper cultivars with the ‘Tsw’ resistance gene provided significant levels of control of disease expression whenever TSW occurred at >4% symptomatic plants in the susceptible check. There was no observable resistance to thrips in any of the cultivars tested. Overall, the top 4 commercial TSW-resistance pepper cultivars for production in decreasing order were ‘Declaration’, ‘Monarcha’, ‘Magico’, and ‘Heritage’ (Table1), but the TSW-susceptible cultivars of ‘Vanguard’, ‘Patriot’, ‘Allegiance’, ‘Aristotle’, ‘Regiment’ and ‘Excursion II’ yielded as well under the disease pressure experienced from 2006 to 2010. Over this period of field testing in Georgia, none of commercial pepper cultivars showed significant susceptibility to the strain of TSWV that we typically have in the Southeast, at least not in the replicated field trials. However, in North Carolina, resistance breaking strains of TSWV have been detected in both commercial as well as research pepper plantings, and are currently under investigation (Kennedy, personal communication). So, significant yield loss in TSW-resistant bell pepper in Georgia has not been reported up to 2012 for commercial plantings of this crop, but has been reported in North Carolina. Thus, the resistance breaking strains of TSWV are not uniformly distributed in the Southeast.
 There are other reported pepper lines with resistance to TSW that were not tested as part of this RAMP project including: Hunter (Bell pepper from Syngenta) Cyrus, Gualda, HA-250, Lord King, Santiago, Sinai, Sir John, Vargas, Zin, Sargon and Hila (Bell peppers from Hazera Genetics), Rioja (Lamuyo pepper from Hazera Genetics), Riata F1, Chesapeake, (Bell pepper from Harris Moran), Mira, and Sirius (Sunbelt Seeds). The field tested lines are excellent producers and provide a high quality pepper under conditions in the southeastern USA.
There are other reported pepper lines with resistance to TSW that were not tested as part of this RAMP project including: Hunter (Bell pepper from Syngenta) Cyrus, Gualda, HA-250, Lord King, Santiago, Sinai, Sir John, Vargas, Zin, Sargon and Hila (Bell peppers from Hazera Genetics), Rioja (Lamuyo pepper from Hazera Genetics), Riata F1, Chesapeake, (Bell pepper from Harris Moran), Mira, and Sirius (Sunbelt Seeds). The field tested lines are excellent producers and provide a high quality pepper under conditions in the southeastern USA.
Authors: Chris Gunter, D. Riley, W. T. Kelley
Use of Reflective Plastic Mulch for Managing TSW
History of mulch trials
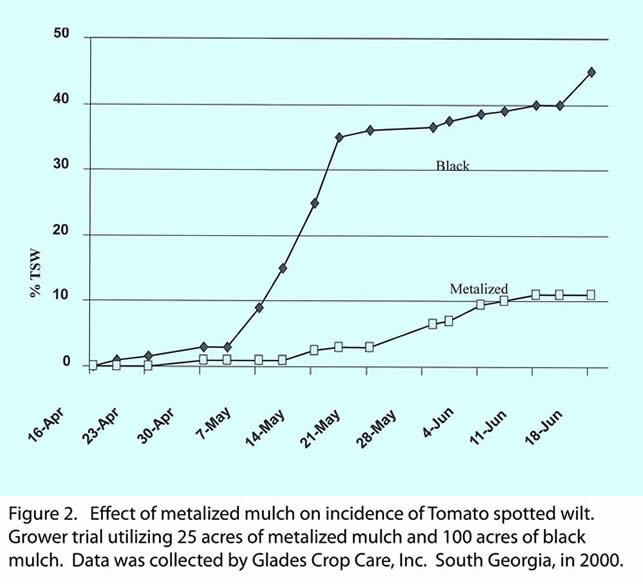
Tomato spotted wilt was first confirmed in the southeast US in 1986 in the panhandle area of north Florida. TSW at first was a fall problem and by 1992 incidence was as high as 80 % in some tomato fields and frequent (3 times weekly) failed to prevent infection. For several years after that incidence was low in fall crops but continued to increase in the spring crop. Research conducted in the mid 90’s showed that primary infection could not be reduced by pesticide sprays but secondary infection could be reduced. In 1997, research was initiated to look at effect of reflective mulches on management of TSW. First reflective mulches were either pigmented silver or painted with silver colored paint. Reflectance of both was low and effect on TSW was minimal. In 1998 metalized (Al metal deposited on polyethylene mulch, see example in Fig. 1) mulch was custom made for research trials and results were very promising. In 1999, 3 grower trials of 1 acre each were conducted with results being very good. Replicated trials at NFREC-Quincy continued with the metalized mulch. Grower trials expanded in 2000 with single grower using about 25 acres of metalized mulch. Results of the trial are shown in Fig. 2. Both replicated field trials (Table 1) and large scale grower trials have shown the benefit of using the metalized mulch for managing TSW.

Metalized mulches work by disorientating the thrips and prevent them from finding the tomato crop. You will still see some infection primarily around the outside of the field. The metalized mulch consists of a very thin (100-300 angstroms) layer of Al deposited on a polyethylene mulch base. It is necessary to make sure that a quality metalized mulch is purchased since in the past there have been problems with the Al layer coming off prematurely, that is, mulch breaking down early and/or mulch integrity such that it cannot be applied to the bed. Good quality metalized mulch should have a reflectance level of 70 % or more. Extensive use of wettable powder formulations of pesticides can discolor the mulch resulting in reduced reflectance. When using fumigants or herbicides under the metalized mulch, caution should be taken to make sure the residues of the materials are at a low enough concentration to allow transplanting without damaging the crop. The thin layer of Al reduces the movement of pesticides through the mulch. With some fumigants, rates can be reduced due to reduction in movement through the mulch. The metalized mulch reduces soil temperatures more than any other mulch due to reflecting sunlight away. At times there has been some yield reduction or delay in harvest due to this cooling effect but does not occur every time with tomatoes. Toward the end of the spring season this cooling effect may actually help the plants grow better. At the beginning of the fall season when soil temperatures are very high, improved stands may occur with the metalized mulch.
Table 1: Effect of mulch types on percentage of plant showing Tomato spotted wilt (values are % TSW) symptoms on specific dates during the growing season. NFREC-Quincy, Spring 2002.
Treatment |
4/25 |
5/7 |
5/17 |
5/29 |
6/10 |
6/20 |
|
||||||
Black |
0.73 a |
3.95 a |
14.94 a |
28.14 a |
38.58 a |
46.61 a |
Metalized |
0.98 a |
2.84 a |
06.72 b |
13.47 b |
20.22 b |
25.09 c |
Heat stripe |
0.84 a |
2.96 a |
06.67 b |
13.90 b |
18.64 b |
23.49 c |
Sonoco printed |
1.24 a |
3.73 a |
09.49 b |
20.00 b |
26.66 b |
35.04 b |
|
||||||
Means within columns separated using Duncan’s multiple range test, 5 % level.
Author: Stephen M. Olson
Use of Plant Activators for Managing TSW
 There is very little that can be done for a tomato or pepper plant that has been inoculated at a very young age with Tomato spotted wilt (TSW) virus. Typically, the virus will induce the disease response of necrotic lesions (spotting) on the leaves within 2-4 weeks after inoculation by viruliferous thrips. If the disease progresses before fruit set, there is usually no commercial yield produced from that plant (Chaisuekul et al. 2003). How quickly the disease symptoms develop depends on temperature and plant maturity at the time of transmission (Riley et al. 2012a). The only commercially available materials which can suppress TSW disease just before or just after inoculation are activators of the plant immune response such as induced systemic resistance (ISR) or systemic acquired resistance (SAR) inducers (Louws et al., 2001; Obradovicet al., 2004; Vavrina et al., 2004). Acibenzolar-S-methyl (ASM) (Actigard 50WG, Syngenta Crop Protection, Greensboro, NC), a structural analog of salicylic acid that induces natural disease resistance (a SAR inducer) has been shown to reduce TSW symptoms (Riley et al. 2012b). The disease reduction level observed under field conditions is usually similar to preventative insecticide sprays targeted at the thrips vectors but can have an additive effect with other control tactics (Awondo et al. 2012).
There is very little that can be done for a tomato or pepper plant that has been inoculated at a very young age with Tomato spotted wilt (TSW) virus. Typically, the virus will induce the disease response of necrotic lesions (spotting) on the leaves within 2-4 weeks after inoculation by viruliferous thrips. If the disease progresses before fruit set, there is usually no commercial yield produced from that plant (Chaisuekul et al. 2003). How quickly the disease symptoms develop depends on temperature and plant maturity at the time of transmission (Riley et al. 2012a). The only commercially available materials which can suppress TSW disease just before or just after inoculation are activators of the plant immune response such as induced systemic resistance (ISR) or systemic acquired resistance (SAR) inducers (Louws et al., 2001; Obradovicet al., 2004; Vavrina et al., 2004). Acibenzolar-S-methyl (ASM) (Actigard 50WG, Syngenta Crop Protection, Greensboro, NC), a structural analog of salicylic acid that induces natural disease resistance (a SAR inducer) has been shown to reduce TSW symptoms (Riley et al. 2012b). The disease reduction level observed under field conditions is usually similar to preventative insecticide sprays targeted at the thrips vectors but can have an additive effect with other control tactics (Awondo et al. 2012).
Effectively, plant resistance to TSW can be induced in genetically susceptible cultivars of tomato by foliar applications of acibenzolar-S-methyl. Momol et al. (2004) demonstrated that such applications are effective in reducing TSW incidence in tomato when applied prior transplant plus every two weeks for six applications. This treatment is useful when planting TSW-susceptible tomato cultivars which may be grown for other horticultural characteristics. The use of Actigard for TSW suppression usually involves spraying just prior to transplant and then at 10-d, 20-d, and 30-d, post-transplant. Acibenzolar-S-methyl rates 2.3, 2.3, 3.5, and 3.5 mg per square meter of treated area are typically applied at transplant, 10-d, 20-d and 30-d after transplant, respectively. The lower rates on younger plants are often used to avoid phytotoxicity effects from the SAR treatment that have been documented in tobacco (Mandal et al. 2008). Also, a delay in fruit maturity (or yield drag) resulting in tomatoes being harvested over a longer period of time has been observed with the use of Actigard in the field (Olson, personal communication). However, total yield is rarely affected.
Authors: D. Langston, F. Louws, D. Riley
Control of Thrips Vectors with Insecticide
 Insecticides are typically the first pest management option growers consider to control damaging insect populations in tomato and pepper. In the Southeast, Lepidopteran pests, such as beet armyworm and tomato fruitworm (corn earworm), and Hemipteran pests, such as stinkbugs, can require frequent applications to avoid economic loss in the late spring and fall growing seasons. These pests are frequently managed with preventive insecticide treatments when blooming begins. These insects are relatively easy to detect because of their large size and the presence of obvious direct feeding damage on the plant. Incidence of plant injury can help scouts key in on pest presence and they can intensify scouting in areas with damage.
Insecticides are typically the first pest management option growers consider to control damaging insect populations in tomato and pepper. In the Southeast, Lepidopteran pests, such as beet armyworm and tomato fruitworm (corn earworm), and Hemipteran pests, such as stinkbugs, can require frequent applications to avoid economic loss in the late spring and fall growing seasons. These pests are frequently managed with preventive insecticide treatments when blooming begins. These insects are relatively easy to detect because of their large size and the presence of obvious direct feeding damage on the plant. Incidence of plant injury can help scouts key in on pest presence and they can intensify scouting in areas with damage.
Thrips are more difficult to scout for because of their tiny size and the direct damage they cause at low populations is obscure. Further, the damage they cause through vectoring virus can appear several weeks after virus transmission, long after a control decision should have been made. Scouting usually requires some type of beat samples (beating plants into a cup or box) or blossom samples. Thrips species (Order: Thysanoptera; Family: Thripidae) that commonly occur in tomato and peppers include: Tobacco thrips (Frankliniella fusca (Hinds)), Western flower thrips (Frankliniella occidentalis (Pergande)), Flower thrips (Frankliniella tritici and Frankliniella bispinosa), and Onion thrips (Thrips tabaci Lindeman). The thrips species that are the most significant pests on tomato are those that are abundant and vector tomato spotted wilt virus (TSWV), which are primarily F. fusca, and F. occidentalis. The flower thrips, Frankliniella tritici, infests tomato annually, but it does not vector TSWV in the field, whereas, Frankliniella bispinosa may vector to a limited extent. Thrips tabaci occurs in relatively low numbers in tomato. The diversity of thrips and differences in injury potential add to the difficulty in managing thrips as proper identification requires examination under magnification by a trained individual.
Spraying for Thrips
Thrips are cryptic in nature, preferring to feed in tight secluded places such as the plant terminal and inside blooms. Because of this, thrips are somewhat protected from insecticide sprays unless good spray coverage into these plant crevices can be attained. A larger problem with thrips treatments has to do with timing of applications. Generally, to avoid direct feeding damage to the crop by thrips, weekly scouting and sprays when thrips exceed five thrips per bloom is sufficient to prevent significant yield loss from direct damage. However, because TSWV transmission can occur at much lower populations and the impact of TSWV transmission in tomato and pepper occurs 2 to 5 weeks after viruliferous thrips feed on the plant, the timing of insecticide applications generally needs to be preventive. Treatments applied at first symptoms of TWSV in a crop have very little chance of impacting disease incidence or severity.
Thrips are quick to infest tomato after transplanting and can transmit the virus within days. Products such as imidacloprid, which interfere with TSWV transmission by interfering with thrips feeding, need to be in the plant prior to or immediately after transplanting or much of the utility of insecticide control may be lost. Contact insecticides with activity against thrips should also be applied during the first four weeks after transplanting to maximize potential reduction of TSWV transmission. Additional sprays after the first four weeks are used to reduce secondary spread of the virus. Once a plant is infected with TSWV, no amount of spraying can save that plant. Also, numerous other plants are probably also already infected as the first symptoms generally do not become apparent for about two weeks after initial infection. Finally, plants infected early in development do not produce any marketable fruit. Thus, preventive applications, particularly early in the season, are a necessity if a grower attempts to manage TSWV with insecticides. Even under ideal conditions with proper insecticide selection and use, if TSWV pressure is even moderate, resistant varieties are the best management option (as long as the resistance remains effective). Insecticidal management can reduce or delay incidence of TSWV but does not eliminate occurrence even under ideal conditions.
Insecticides Recommended for Thrips Control
At-planting neonicatinoid insecticides, such as imidacloprid, which have anti-feeding effects on tobacco thrips, are recommended as a tray drench, transplant drench, or early drip injection treatment and have provided consistent reductions in TSWV infection in susceptible varieties. Early season (first four weeks post-transplant) foliar insecticide treatments of products with known efficacy against thrips can also have a beneficial effect in reducing the incidence of this disease. Older chemistries such as methamidophos (Monitor), lambda-cyhalothrin (Warrior) and methomyl (Lannate) may have State/local pesticide restrictions or limitations, and their labels should be closely checked prior to use (as should all pesticides).Insecticide resistance is known to occur in thrips and thrips species composition tends to change over the season. Thus, insecticide selection should include rotations to best target the appropriate thrips species for a given insecticide and to delay selection for resistance. For example, imidacloprid is probably best used near transplanting, to target feeding inhibition in tobacco thrips, combined with foliar treatments of insecticides with long pre-harvest interval (PHI) that are effective against western flower thrips used during the first four weeks. Insecticides with shorter PHIs and different modes of action (MOA) than insecticides used during the first four weeks should be used after flowering to harvest.
Finally, bear in mind that TSWV-resistant tomato and pepper can be highly effective for controlling the disease. In these situations, the main benefit from insecticide use is in controlling middle to late season damage to the flowers and fruit which is primarily caused by flower thrips. Thus, when planting crops highly resistant to TSWV, the main use of insecticides for thrips is controlling direct thrips feeding damage to the fruit which is caused by flower thrips and only those products effective against Flower thrips should be used. Again rotating insecticide MOAs is highly recommended. Any MOA should be limited to a single thrips generation (2-3 weeks in hot weather) and then avoided for at least a generation.
Insecticide Efficacy Against Thrips in Tomato/Pepper in the Southeast |
|||||
Insecticide |
Efficacy Rating 1 |
Registration |
MOA |
||
Tobacco thrips |
Flower thrips |
Tomato |
Pepper |
||
Acephate (e.g. Orthene 97) |
G |
G |
No 2 |
Yes |
1B |
Dinotefuran (e.g. Venom 70SG) |
G |
G |
Yes |
Yes |
4A |
Endosulfan(e.g. Endosulfan 3EC) 3 |
F |
F |
Yes |
Yes |
2A |
Imidacloprid (e.g. Admire 2F and Pro 4.6F) |
G-AF |
P |
Yes |
Yes |
4A |
Methomyl (e.g. Lannate 2.4LV and 90SP) |
G |
G |
Yes |
Yes |
1A |
Oxamyl (e.g. Vydate 2L) |
G |
G |
Yes |
Yes |
1A |
Spinetoram (e.g. Radiant 1SC) |
G-E |
G-E |
Yes |
Yes |
5 |
Pyrethroid insecticides (see footnote 4) |
G |
F-P |
Yes |
Yes |
3 |
- Efficacy rating: E=excellent, G=good, F=fair, P=poor, AF=anti-feeding effect
- Individual products may not be labeled for the specific pest but should provide indicated level of control when applied according to label instructions for the crop.
- Endosulfan is labeled through 2015 on pepper and tomato.
- labeled pyrethroid insecticides include bifenthrin (e.g. Capture 2EC), lambda-cyhalothrin (e.g.Warrior 1CS), permethrin (e.g. Pounce 3.2EC), zeta-cypermethrin (e.g. MustangMax 0.8EC), esfenvalerate (e.g. Asana XL), cyfluthrin (e.g. Tombstone 2EC, Baythroid XL)
Authors: Stormy Sparks, David Riley
Forecasting Thrips and TSW
The TSWV risk tool is accessed online (see 'TSW Risk Estimator' link above) as a webpage through which visitors are able to request risk estimates. By supplying a small amount of information required for estimation (location, and historical disease incidence), a user prompts the tool to output risk information that is as specific to the user’s situation as we can make it. A description of the user’s situation can be broken into two parts: there are descriptors that each relate to disease risk in one way throughout the region, and descriptors that are either unobservable or currently unknown and may differ among growers and locations. Descriptors that relate to disease risk consistently across the region are generally driven by weather, specifically, temperature and precipitation. Research has shown that the relationships between weather and disease risk are not all direct, and that prediction is improved by understanding how weather affects different components of the disease system. For example, precipitation during the spring is understood to cause mortality in thrips and thus decrease later thrips numbers, but precipitation at this time is also understood to promote growth of plants that harbor inoculum and thus increase later disease risk.
The tool computes a relative risk estimate using weather data. The user inputs a location to which the estimate should apply, and the tool begins by querying weather records (from the NC State Climate Office’s database) for that location. Temperature records come from nearby weather stations. Precipitation is estimated using a combination of Doppler radar measurement, and nearby rain gauges – this method, called Multi-Sensor Precipitation Estimation (MPE), allows for precipitation to be estimated specifically for the user’s location. Temperature records from the preceding two years are queried, and used in two ways: 1) as part of a calculation to describe the expected development of thrips, as a function of cumulative heat, where warmer conditions lead to more thrips, and 2) directly, to describe a general relationship between the severity of mildness of a winter as an effect on the disease system overall, where warmer conditions mean more disease risk. Precipitation estimates are also used in two ways: 1) as part of the calculation to describe thrips development during the winter, and 2) directly, as a description of general favorability to plant growth during the spring. What results is a quantitative description of conditions, where previous-year thrips numbers and current-year favorability to thrips and plant growth are positively correlated with disease risk, but where current-year rainfall reduces the influence of previous-year thrips presumably by suppressing their activity.
After computing this relative estimate of disease risk using weather data, the tool renders it in terms of the user’s entered disease history. This entry of disease history allows the tool to base estimates on effects that are understood to be general, and consistent across locations, by relating these estimates for preceding years to the user’s own history. For example, in a location where conditions vary minimally year-to-year, and disease risk is typically low, the tool will estimate relative risk for the preceding five years, and these estimates will also vary minimally. The user inputs a low value for historical risk (8%), and the tool calibrates its output based on the correction necessary to equate the average of historical estimates to the user’s input of historical risk. Because the location’s weather conditions are not highly variable, the risk estimate will likely be close to the historical risk entered by the user. If the location were one with more variation in weather conditions year-to-year, then the difference between the historical risk entered by the user, and the risk estimate output by the tool for the current year, can differ to a degree informed by the variation in weather conditions.
The referenced calibration of output occurs through a function that constrains outputs to the real range of zero to one hundred percent, and this function is based on our understanding of the spread of disease through populations of hosts. For a user whose typical disease incidence is high, the amount by which incidence could decrease this year is high, but the amount by which it could increase is low. Thus, the current year’s risk estimate is not relative to the historical average in percentage units (i.e. “ten percent less than typical”) because at high or low typical incidence levels, or for unusually high variations in risk conditions, this would often cause estimation to unrealistically be for zero or complete disease risk. Instead, the relationship is between the relative disease estimate and the degree to which incidence is able to change in one or the other direction, given the typical incidence entered by the user.
Lastly, the tool computes estimates of end-of-season disease incidence – the percentage of plants infected at the end of the season – and is available during the spring. An estimate can be computed as early as April 1, and is updated as time passes until May 31. Updates are based on the weather that is observed after April 1, understood to affect the abundance and activity of thrips during that time. For example, an estimate of “lower than usual” at April 1 will increase during the spring if the spring is warmer and drier than usual, and thus conducive of thrips population growth and activity. At May 31, the trajectory leading to end-of-season incidence is set for estimation purposes, and is not further updated.
Author: Thomas Chappell
References
Tomato Host Plant Resistance References
Boiteux, L.S. and L. de B. Giordano. 1993. Genetic basis of resistance against two Tospovirus species in tomato (Lycopersicon esculentum). Euphytica 71:151-154.
Brommonschenkel, S.H. and S.D. Tanksley. 1997. Map-based cloning of the tomato genomic region that spans the Sw-5 tospovirus resistance gene in tomato. Mol. Gen. Genet. 256:121-126.
Canady, M.A., M.R. Stevens, M.S. Barineau, and J.W. Scott. 2001. Tomato spotted wilt virus (TSWV) resistance in tomato derived from Lycopersicon chilense Dun. LA 1938. Euphytica. 117:19-25.
Cho, J.J., D.M. Custer, S.H. Brommonschenkel, and S.D. Tanksley, 1996. Conventional breeding: host- plant resistance and the use of molecular markers to develop resistance to tomato spot wilt virus in vegetables. Acta Hort. 431: 367-378.
Clayberg, C. D., L. Butler, C.M. Rick, and P. A. Young. 1960. Second list of known genes in the tomato. J. Hered. 51:167-174.
Finlay, K.W. 1953. Inheritance of spotted wilt resistance in tomatoes; five genes controlling spotted wilt resistance in four tomatoes. Aust. J. Biol. Sci. 6:153-163.
Folkertsma, R.T., M.I. Spassova, M. Prins, M.R. Stevens, J. Hille, and R. W. Goldbach. 1999. Construction of a bacterial artificial chromosome (BAC) library of Lycopersicon esculentum cv. Stevens and its application to physically map the Sw-5 locus. Molecular Breeding 5:197-207.
Garland, S., M. Sharman, D. Persley, and D. McGrath. 2005. The development of an improved PCR-based marker system for Sw-5, and important TSWV resistance gene of tomato. Australian Journal of Ag Res. 56(3):285-289.
Gubba, A., C. Gonsalves, M.R. Stevens, D.M. Tricoli, and D. Gonsalves. 2002. Combining transgenic and natural resistance to obtain broad resistance to tospovirus infection in tomato (Lycopersicon esculentum Mill.). Molecular Breeding 9:13-23.
Jones, R.A.C., R. Koenig, and D.E. Lesemann. 1980. Pepino mosaic virus, a new potexvirus from pepino Solanum muricatum. Ann. of Appl. Biol.94:61-68.
Kim, J.W., S.S.M. Sun, and T.L. German. 1994. Diseases resistance in tobacco and tomato plants transformed with the tomato spotted wilt virus nuclesocapsid gene. Plant Dis. 6:615-621.
Latham, L.J. and R.A.C. Jones. 1998. Selection of resistance breaking strains of tomato spotted wilt tospovirus. Ann. Appl. Biol. 133:385-402.
Moon, H., and J.S. Nicholson. 2007. AFLP and SCAR markers linked to tomato spotted wilt virus resistance in tobacco. Crop Science 47(5):1887-1894.
Paterson, R.G., S.J. Scott, and R.C. Gergerich. 1989. Resistance in two Lycopersicon species to an Arkansas isolate of tomato spotted wilt virus. Euphytica 43:173-178.
Price, D.L., F.D. Memmott, J.W. Scott, S.M. Olson, and M.R. Stevens. 2007. Identification of molecular markers linked to a new tomato spotted wilt virus resistance source in tomato. Rept. Tomato Genet. Coop. 57:35-36.
Roselló, S., B. Ricarte, M. Jose Diez, F. Nuez. 2001. Resistance to tomato spotted wilt virus introgressed from Lycopersicon peruvianum in line UPV 1 may be allelic to Sw-5 and can be used to enhance the resistance of hybrids cultivars. Euphytica. V. 119. 3:357-367(11).
Roselló, S., S. Soler, M.J. Díez, J.L. Rambla, C. Richarte, and F. Nuez. 1999. New sources for high resistance of tomato to the tomato spotted wilt virus from Lycopersicon peruvianum. Plant Breeding 118:425-429.
Roselló, S., M. J. Diez, A. Lacasa, C. Jordá, and F. Nuez. 1997. Testing resistance to TSWV introgressed from Lycopersicon peruvianum by artificial transmission techniques. Euphytica 98:93-98.
Roselló, S., J. Diez, and F. Nuez. 1998. Genetics of tomato spotted wilt virus resistance coming from Lycopersicon peruvianum. Eur. J. Plant Pathol. 104:499-509.
Saidi, M. and S.D. Warade. 2008. Tomato breeding for resistanct to tomato spotted wilt virus (TSWV): an overview of conventional and molecular approaches. Czech Journal of Genetics and Plant Breeding 44(3):83-92.
Scott, J.W., M.R. Stevens, and S. M. Olson. 2005. An alternative source of resistance to tomato spotted wilt virus. Rept. Tomato Genet. Coop. 55:40-42.
Scott, J.W., S. F. Hutton, S. M. Olson, and M.R. Stevens 2011. Spotty results in our Sw-7 tomato spotted wilt virus research.
Soler S., J. Cebolla-Cornejo, and F. Nuez. 2003. Control of diseases induced by tospoviruses in tomato: An update of the genetic approach. Phytopathologia Mediterranea 42(3):207-219
Stevens, M.R., D.K. Heiny, P.D. Griffiths, J.W. Scott and D.D. Rhoads. 1996. Identification of co-dominant RAPD markers tightly linked to the tomato spotted wilt virus (TSWV) resistance gene Sw-5 Rept. Tomato Genet. Coop. 46:27.
Stevens, M.R., E.M. Lamb, and D.D. Rhoads. 1995. Mapping the Sw-5 locus for tomato spotted wilt virus resistance in tomatoes using RAPD and RFLP analyses. Theor. Appl. Genet. 90:451-456.
Stevens, M. R., S.J. Scott, and R.C. Gergerich. 1992. Inheritance of a gene for resistance to tomato spotted wilt virus (TSWV) from Lycopersicon peruvianum Mill. Euphytica 59:9-17.
Tanksley, S. D., M.W. Ganal, J.P. Prince, M.C. de Vincente, M.W. Bonierbale, P. Broun, T. M. Fulton, J. J. Giovannoni, S. Grandillo, G.B. Martin, R. Messeguer, J. C. Miller, L. Miller, A.H. Paterson, O. Pineda, M.S. Roder, R. A. Wing, W.Wu, and N. D. Young. 1992. High-density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141-1160.
Thompson, G.J. and J.J.B. van Zijl. 1996. Control of tomato spotted wilt virus in tomatoes in South Africa. Acta Hort. 431:379-384.
van Zijl, J.J.B., S.E. Bosch and C.P.J. Coetzee. 1986. Breeding tomatoes for processing in South Africa. Acta Hort. 194:69-75.
Pepper Host Plant Resistance References
Beaudoin, A. L., N. D. Kahn, and G. G. Kennedy. 2009. Bell and banana pepper exhibit mature-plant resistance to tomato spotted wilt Tospovirus transmitted by Frankliniella fusca (Thysanoptera: Thripidae). J. Econ. Entomol. 102: 30-35.
Black, L. L., H. A. Hobbs, and J. M. Gatti. 1991. Tomato spotted wilt virus resistance in Capsicum chinensePI152225 and 159236. Plant Dis. 75: 863.
Black, L. L., H. A. Hobbs and D. S. Kammerlohr. 1996. Resistance of Capsicum chinense lines to tomato spotted wilt virus from Louisiana, USA, and inheritance of resistance. Acta Hortic. 431: 393-401.
Boiteux, L. S. 1995. Allelic relationships between genes for resistance to tomato spotted wilt tospovirus in Capsicum chinense. Theor. Appl. Genet. 90: 146-149.
Boiteux, L. S., and T. Nagata. 1992. Susceptibility of Capsicum chinense PI 159236 to tomato spotted wilt virus isolates in Brazil. Plant Dis. 77: 210.
Boiteux, L. S., and A. C. de Avila. 1994. Inheritance of a resistance specific to tomato spotted wilt tospovirus in Capsicum chinense ‘PI 159236’. Euphytica 75: 139-142.
Boiteux, L. S, T. Nagata, W. P. Dutra, and M. E. N. Fonseca. 1993. Sources of resistance to tomato spotted wilt virus (TSWV) in cultivated and wild species of Capsicum. Euphytica 67: 89-94.
Costa, J., M. S. Catalá, A. Lacasa, M. J. Díez, and F. Nuez. 1995. Introduction of plant genetic resistance to TSWV from C. chinense ‘PI 159236’ in different pepper genetic backgrounds. In First International Symposium on Solanacea for Fresh Market. March 28–31 1995, Malaga, Spain. Acta Hortic. 412: 523–532.
Genda, Y., S. Tsuda, O. Nunomura, and T. Ito. 2008. Development of an assay system using thrips-mediated inoculation to evaluate resistance of Capsicum spp. to Tomato spotted wilt virus. J. Gen. Plant Pathol. 74: 171-175.
Gitaitis, R. D., C. C. Dowler, and R. B. Chalfant. 1998. Epidemiology of tomato spotted wilt in pepper and tomato in Southern Georgia. Plant Dis. 82: 752-756.
Hobbs, H. A., L. L. Black, R. R. Johnson, and R. A. Valverde. 1994. Differences in reactions among tomato spotted wilt virus isolates to three resistant Capsicum chinense lines. Plant Dis. 78: 1220.
Lopez, C., J. Aramburu, L. Galipienso, S. Soler, F. Nuez and L. Rubio. 2011. Evolutionary analysis of tomato Sw-5 resistance breaking isolates of Tomato spotted wilt virus. Journal of General Virology 92: 210–215.
Margaria, P., M. Ciuffo, and M. Turina. 2004. Resistance breaking strains of Tomato spotted wilt virus (Tospovirus-Bunyaviridae) on resistant pepper cultivars in Almeria (Spain). Plant Pathol. 53: 795.
Moury, B., A. Palloix, K. Selassie-Gebre, and G. Marchoux. 1997. Hypersensitive resistance to tomato spotted wilt virus in three Capsicum chinense accessions is controlled by a single gene and is overcome by virulent strains. Euphytica 94: 45-52.
Moury, B., K. Selassie-Gebre, G. Marchoux, A. M. Daubeze, and A. Palloix. 1998. High temperature effects on hypersensitive resistance to tomato spotted wilt tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 104: 489-498.
Persley, D. M., J. E. Thomas, and M. Sharman. 2006. Tospoviruses - an Australian perspective. Australas. Plant Path. 35: 161–180.
Roggero, P., V. Lisa, G. Nervo, and S. Pennazio. 1996. Continuous high temperature can break the hypersensitivity of Capsicum chinense 'PI152225' to tomato spotted wilt tospovirus (TSWV). Phytopathologia Mediterranea 35: 117-120.
Roggero, P., V. Masenga, and L. Tavella. 2002. Field isolates of Tomato spotted wilt virus overcoming resistance in pepper and their spread to other hosts in Italy. Plant Dis. 86: 950-954.
Sharman, M., and D. M. Persley. 2006. Field isolates of Tomato spotted wilt virus overcoming resistance in Capsicum in Australia. Australas. Plant Path. 35: 123-128.
Soler, S., M. J. Diez, and F. Nuez. 1998. Effect of temperature regime and growth stage interaction on pattern of virus presence in TSWV-resistant accessions of Capsicum chinense. Plant Dis. 82: 1199-1204.
Plant Activator References:
Awondo, S. N., E. G.Fonsah, D. Riley and M. Abney. 2012. Effectiveness of Tomato-spottedwilt virus management tactics. J. Econ. Entomol. 105: 943-948.
Chaisuekul, C., D. Riley, and H. Pappu. 2003. Transmission of Tomato spotted wilt virus to tomato plants of different ages. J. Entomol. Sci. 38: 126-135.
Louws, F.J., Wilson, M., Campbell, H.L., Cuppels, D.A., Jones, J.B., Shoemaker, P.B., Sahin, F., Miller, S.A., 2001. Field control of bacterial spot and bacterial speck of tomato using plant activator. Plant Dis. 85, 481–488.
Mandal, B., S. Mandal, A. S. Csinos, N. Martinez, A. K. Culbreath and H. R. Pappu. 2008. Biological and molecular analyses of the acibenzolar-S-methyl-induced systemic acquired resistance in flue-cured tobacco against tomato spotted wilt virus. Phytopathology 98: 196–204.
Momol, M. T., S. M. Olson, J. E. Funderburk, J. Stavisky, and J. J. Marois. 2004. Integrated management of tomato spotted wilt on field-grown tomatoes. Plant Dis. 88: 882–890.
Obradovic, A., Jones, J.B., Momol, M.T., Balogh, B., Olson, S.M., 2004. Management oftomato bacterial spot in the field by foliar applications of bacteriophages andSAR inducers. Plant Dis. 88, 736–740.
Riley, D.G. S. V. Joseph, R. Srinivasan. 2012a. Temporal relationship of thrips populations to Tomato spotted wilt incidence in tomato in the field. J. Entomol. Sci. 47: 65-75.
Riley, D.G. S. V. Joseph, R. Srinivasan. 2012b. Reflective Mulch and Acibenzolar-S-methyl treatments relative to thrips (Thysanoptera: Thripidae) and Tomato spotted wilt virus incidence in tomato. J. Econ. Entomol. (in press).
Vavrina, C.S., Roberts, P.D., Kokalis-Burelle, N., Ontermaa, E.O., 2004. Systemicresistance in tomato: Greenhouse screening of commercial products andapplication programs. HortScience 39, 433–437.
